CMaT Researchers Introduce Novel Supply Chain and Process Modeling Algorithms, Methods, and Tools for Cell Therapy Manufacturing and Distribution
Outcome/Accomplishment
The Center for Cell Manufacturing Technologies (CMaT), headquartered at the Georgia Institute of Technology, developed several key resources to improve cell therapy manufacturing and distribution. The innovations at the National Science Foundation Engineering Research Center (NSF-ERC) included digital models of a centralized and a distributed cell manufacturing and distribution network; a capacity planning tool that selects the optimal locations and capacities of manufacturing facilities; and hybrid cost analysis models capable of activity-based costing and parametric costing to assess the return-on-investment of cell manufacturing technologies.
Impact/Benefits
In order for living cell therapies to advance into broad healthcare use, cells will have to be produced in much larger quantities and with more consistent quality than is now possible. Additionally, supply chain challenges in biomanufacturing commonly include balancing affordability, responsiveness, transparency, security (free of physical and cyber disruptions), and resilience with patient access, managing personalized products, and the dynamic, living, nature of the products in the supply chain. A robust set of analytical tools will be needed to help meet these challenges by enabling industry and clinical facilities to pursue consistent production of safe and efficacious cell therapy products at low cost. For example, work on scaling cell production will enable manufacturing of sufficient numbers of cells to replace damaged organs, such as during the loss of heart muscle after a heart attack, at a cost that makes these therapies accessible to broad segments of society. The novel supply chain and process modeling algorithms, methods, and tools developed recently at CMaT will enable greater efficiencies in the supply chain, storage, and distribution systems for these therapeutic cell products.
Explanation/Background
The Georgia Tech Manufacturing Institute (GTMI) partnered with CMaT, the Marcus Center for Therapeutic Cell Characterization and Manufacturing (MC3M), the Institute for Data Engineering and Science, and BioFab USA to develop a customizable and interactive supply chain simulation program and decision support system to facilitate informed and dynamic supply chain modeling, tailored to the unique needs of the biomanufacturing industry.
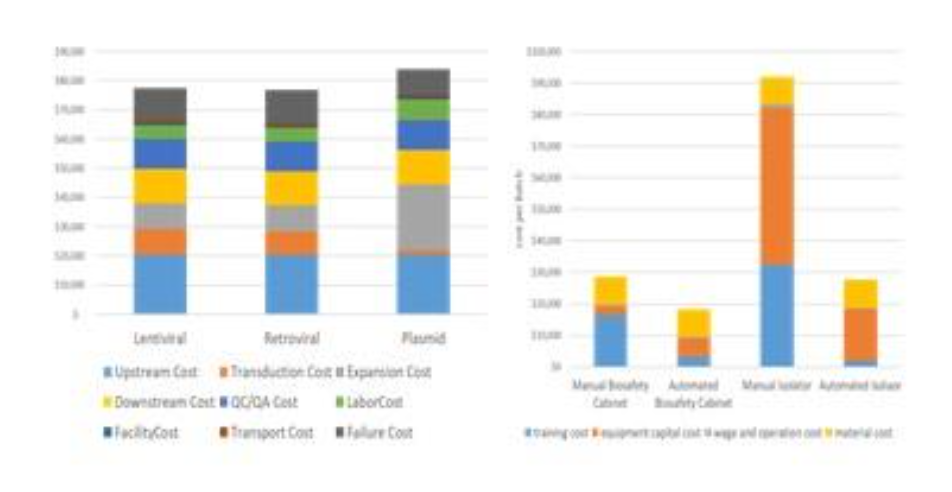
CMaT research led by Wang et al. included the design of a computation experiment for a comparison study of three supply chain network structures. Additional work investigated impacts of reagent supply disruptions and labor shortages on patient access and capacity utilization. Chimeric antigen receptors (CAR)-T cell therapies were used as an exemplar product. The team modeled response surfaces of patient mortality versus reagent availability under FIFO policy in a disruption duration of 9 months and completed case studies of cost analysis. Wang's findings have been published in three journal articles appearing in American Pharmaceutical Review; Cell and Gene Therapy Insights; and Cytotherapy.
The supply chain logistics (SCL) solution developed by CMaT and its partners at GTMI directly addresses national connectivity and secure data sharing among key players throughout the medical biomanufacturing supply chain, to allow for more responsive and informed supply chain decision making. Users will have the ability to securely upload their own test data to "plug and play" software modules relevant to their test cases and then to review the output of their simulations to inform supply chain planning. The software platform has already been tested by several academic and industry partners and received very positive reviews. GTMI is now working on the vision for version 2.0 of this platform, to make it even more useful and more broadly available.
Location
Atlanta, GeorgiaStart Year
Advanced Manufacturing
Advanced Manufacturing
Lead Institution
Core Partners
Fact Sheet
Outcome/Accomplishment
The Center for Cell Manufacturing Technologies (CMaT), headquartered at the Georgia Institute of Technology, developed several key resources to improve cell therapy manufacturing and distribution. The innovations at the National Science Foundation Engineering Research Center (NSF-ERC) included digital models of a centralized and a distributed cell manufacturing and distribution network; a capacity planning tool that selects the optimal locations and capacities of manufacturing facilities; and hybrid cost analysis models capable of activity-based costing and parametric costing to assess the return-on-investment of cell manufacturing technologies.
Location
Atlanta, GeorgiaStart Year
Advanced Manufacturing
Advanced Manufacturing
Lead Institution
Core Partners
Fact Sheet
Impact/benefits
In order for living cell therapies to advance into broad healthcare use, cells will have to be produced in much larger quantities and with more consistent quality than is now possible. Additionally, supply chain challenges in biomanufacturing commonly include balancing affordability, responsiveness, transparency, security (free of physical and cyber disruptions), and resilience with patient access, managing personalized products, and the dynamic, living, nature of the products in the supply chain. A robust set of analytical tools will be needed to help meet these challenges by enabling industry and clinical facilities to pursue consistent production of safe and efficacious cell therapy products at low cost. For example, work on scaling cell production will enable manufacturing of sufficient numbers of cells to replace damaged organs, such as during the loss of heart muscle after a heart attack, at a cost that makes these therapies accessible to broad segments of society. The novel supply chain and process modeling algorithms, methods, and tools developed recently at CMaT will enable greater efficiencies in the supply chain, storage, and distribution systems for these therapeutic cell products.
Explanation/Background
The Georgia Tech Manufacturing Institute (GTMI) partnered with CMaT, the Marcus Center for Therapeutic Cell Characterization and Manufacturing (MC3M), the Institute for Data Engineering and Science, and BioFab USA to develop a customizable and interactive supply chain simulation program and decision support system to facilitate informed and dynamic supply chain modeling, tailored to the unique needs of the biomanufacturing industry.
CMaT research led by Wang et al. included the design of a computation experiment for a comparison study of three supply chain network structures. Additional work investigated impacts of reagent supply disruptions and labor shortages on patient access and capacity utilization. Chimeric antigen receptors (CAR)-T cell therapies were used as an exemplar product. The team modeled response surfaces of patient mortality versus reagent availability under FIFO policy in a disruption duration of 9 months and completed case studies of cost analysis. Wang's findings have been published in three journal articles appearing in American Pharmaceutical Review; Cell and Gene Therapy Insights; and Cytotherapy.
The supply chain logistics (SCL) solution developed by CMaT and its partners at GTMI directly addresses national connectivity and secure data sharing among key players throughout the medical biomanufacturing supply chain, to allow for more responsive and informed supply chain decision making. Users will have the ability to securely upload their own test data to "plug and play" software modules relevant to their test cases and then to review the output of their simulations to inform supply chain planning. The software platform has already been tested by several academic and industry partners and received very positive reviews. GTMI is now working on the vision for version 2.0 of this platform, to make it even more useful and more broadly available.