ERC Pioneers Genetic Modification of Transplant Tissues Outside of the Body
Outcome/Accomplishment
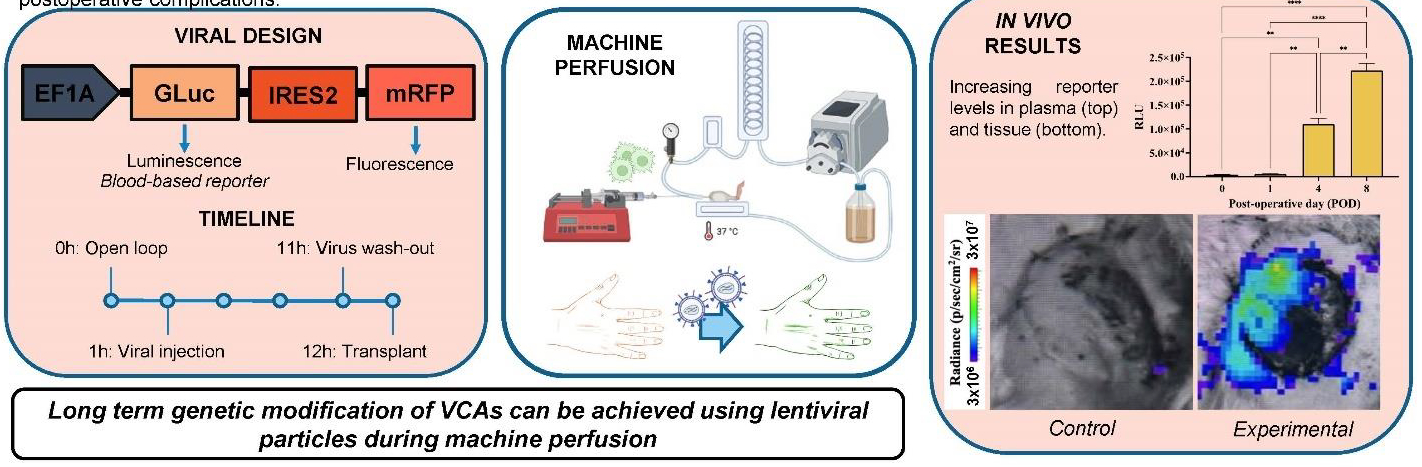
The NSF-funded Engineering Research Center (ERC) for Advanced Technologies for the Preservation of Biological Systems (ATP-Bio), co-led by the University of Minnesota and Massachusetts General Hospital, demonstrated for the first time the ability to genetically modify Vascularized Composite Allografts (VCA)—in this case rat hindlimbs—and successfully transplant them into rats.
Impact/Benefits
Recipients of donated organs and tissues must have their immune systems suppressed with powerful drugs for life to prevent their bodies from rejecting organs. ATP-Bio's technology could lead to "smart grafts" containing genetic reporters that would detect rejection without the invasive biopsies that are the gold standard today. Alternatively, the grafts could contain genetic code for proteins that tamp down on inflammation, which might prevent rejection entirely.
Explanation/Background
VCA transplants are complex; they involve multiple tissue types including skin, nerves, and blood vessels. In the new process, developed in the lab of Korkut Uygun at Massachusetts General Hospital and colleagues, genetic modification takes place when the VCA is no longer connected to its donor but not yet grafted to its recipient.
A perfusion machine is used to continuously pump a special solution into the VCA, mimicking blood flow. Injected into the solution are particles of a virus designed to deliver genetic material to the VCA organ. Transplantation takes place after removing the virus.
Location
Minneapolis, Minnesotawebsite
Start Year
Biotechnology and Healthcare
Biotechnology and Healthcare
Lead Institution
Core Partners
Fact Sheet
Outcome/Accomplishment
The NSF-funded Engineering Research Center (ERC) for Advanced Technologies for the Preservation of Biological Systems (ATP-Bio), co-led by the University of Minnesota and Massachusetts General Hospital, demonstrated for the first time the ability to genetically modify Vascularized Composite Allografts (VCA)—in this case rat hindlimbs—and successfully transplant them into rats.
Location
Minneapolis, Minnesotawebsite
Start Year
Biotechnology and Healthcare
Biotechnology and Healthcare
Lead Institution
Core Partners
Fact Sheet
Impact/benefits
Recipients of donated organs and tissues must have their immune systems suppressed with powerful drugs for life to prevent their bodies from rejecting organs. ATP-Bio's technology could lead to "smart grafts" containing genetic reporters that would detect rejection without the invasive biopsies that are the gold standard today. Alternatively, the grafts could contain genetic code for proteins that tamp down on inflammation, which might prevent rejection entirely.
Explanation/Background
VCA transplants are complex; they involve multiple tissue types including skin, nerves, and blood vessels. In the new process, developed in the lab of Korkut Uygun at Massachusetts General Hospital and colleagues, genetic modification takes place when the VCA is no longer connected to its donor but not yet grafted to its recipient.
A perfusion machine is used to continuously pump a special solution into the VCA, mimicking blood flow. Injected into the solution are particles of a virus designed to deliver genetic material to the VCA organ. Transplantation takes place after removing the virus.