Nanoscale Tandem Catalyst Boosts Yields in Oxidative Dehydrogenation of Propane
Outcome/Accomplishment
A new nanoscale tandem catalyst gave world-leading yields of propylene during oxidative dehydrogenation of propane in research at the Center for Innovative and Strategic Transformation of Alkane Resources (CISTAR), an NSF-funded Engineering Research Center (ERC) based at Purdue University. Propane is a significant component of natural gas liquids produced in abundance from distributed shale gas resources.
Impact/Benefits
The new catalyst helps to mitigate the diminishing effectiveness of oxidative dehydrogenation of propane to propylene at high yields because propylene itself is more readily oxidized than propane. In improving the oxidative dehydrogenation of propane to propylene, the catalyst could have a significant impact on energy efficient production of this chemical intermediate.
Explanation/Background
Propylene is an essential intermediate for the production of materials, chemicals, and fuels. Oxidative dehydrogenation (ODH) of light alkanes can yield olefins like propylene with much lower energy inputs than is required by existing, non-oxidative routes, thus reducing GHG emissions and costs. ODH may also yield novel process configurations requiring lower capital investments that are more suitable for distributed manufacturing.
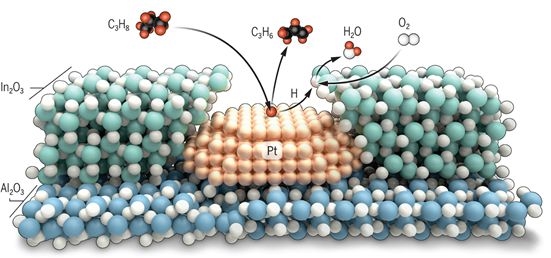
Specifically, atomic layer deposition was used to grow a 2-nanometer shell of indium oxide over a platinum nanoparticle catalyst. This nanostructure efficiently couples the propane dehydrogenation reaction over platinum with the selective hydrogen oxidation reaction over indium oxide, thereby minimizing the undesired total combustion of propane. Other nanostructured arrangements of platinum and indium oxide lead to propane combustion because they cannot organize the desired reactions sequentially. The net effect of the new catalyst is rapid and stable ODH at high yields exceeding those possible in the absence of oxygen and without complex and costly reactor designs. Tandem catalysis using this nanoscale overcoating geometry is validated as an opportunity for highly selective catalytic performance in a grand challenge reaction.
Location
West Lafayette, Indianawebsite
Start Year
Energy and Sustainability
Energy, Sustainability, and Infrastructure
Lead Institution
Core Partners
Fact Sheet
Outcome/Accomplishment
A new nanoscale tandem catalyst gave world-leading yields of propylene during oxidative dehydrogenation of propane in research at the Center for Innovative and Strategic Transformation of Alkane Resources (CISTAR), an NSF-funded Engineering Research Center (ERC) based at Purdue University. Propane is a significant component of natural gas liquids produced in abundance from distributed shale gas resources.
Location
West Lafayette, Indianawebsite
Start Year
Energy and Sustainability
Energy, Sustainability, and Infrastructure
Lead Institution
Core Partners
Fact Sheet
Impact/benefits
The new catalyst helps to mitigate the diminishing effectiveness of oxidative dehydrogenation of propane to propylene at high yields because propylene itself is more readily oxidized than propane. In improving the oxidative dehydrogenation of propane to propylene, the catalyst could have a significant impact on energy efficient production of this chemical intermediate.
Explanation/Background
Propylene is an essential intermediate for the production of materials, chemicals, and fuels. Oxidative dehydrogenation (ODH) of light alkanes can yield olefins like propylene with much lower energy inputs than is required by existing, non-oxidative routes, thus reducing GHG emissions and costs. ODH may also yield novel process configurations requiring lower capital investments that are more suitable for distributed manufacturing.
Specifically, atomic layer deposition was used to grow a 2-nanometer shell of indium oxide over a platinum nanoparticle catalyst. This nanostructure efficiently couples the propane dehydrogenation reaction over platinum with the selective hydrogen oxidation reaction over indium oxide, thereby minimizing the undesired total combustion of propane. Other nanostructured arrangements of platinum and indium oxide lead to propane combustion because they cannot organize the desired reactions sequentially. The net effect of the new catalyst is rapid and stable ODH at high yields exceeding those possible in the absence of oxygen and without complex and costly reactor designs. Tandem catalysis using this nanoscale overcoating geometry is validated as an opportunity for highly selective catalytic performance in a grand challenge reaction.