Scalable Manufacturing of iPSC-derived Cardiac Cells
Outcome/Accomplishment
Researchers at the U.S. National Science Foundation (NSF)-funded Cell Manufacturing Technologies (NSF CMaT) Engineering Research Center (ERC), have developed a novel technique for generating cardiac cells derived from induced pluripotent stem cells (iPSCs). The team successfully identified genetic markers of atrial and ventricular cardiac fibroblast to enable discrimination of these populations. Right ventricular cardiomyocytes were then generated from iPSCs and validated for their molecular and functional properties. Finally, the team generated iPSC-derived pericytes from cardiac progenitor cells using constitutive activation of NOTCH1 signaling.
Impact/Benefits
: Cell therapies, especially stem cell and immune cell therapies, are revolutionizing the way we treat devastating, incurable, and chronic diseases, such as heart disease. Human-induced iPSCs hold great promise for reducing the mortality of cardiovascular disease by cellular replacement of infarcted cardiomyocytes (CMs). NSF CMaT’s work on iPSC-derived cardiac cells will impact how manufacturing develops in early-stage clinical implementation of these cells and other cell types that require differentiation. Specifically, NSF CMaT research enhances the scalability of manufacturing iPSC-derived cardiac cells and microtissues and improves the quality and maturity of the resulting cells for in vitro diagnostic and in vivo therapeutic applications.
Industry partners have already expressed interest in a variety of iPSC-derived cell types. Efforts to generate chamber-specific cell types are now underway, including atrial and ventricular cardiomyocytes and cardiac fibroblasts. While the focus of this work has been on cardiac cells primarily, the approaches and technologies developed will be relevant to other iPSC-derived cell types and iPSC-derived products, including iPSC-derived immune cells and neural cells.
Explanation/Background
The NSF CMaT research team, led by Sean Palecek at the University of Wisconsin-Madison, made significant progress in using materials-based approaches to improve the differentiation and maturation of iPSC-CMs. To generate cardiac pericyte-like cells from iPSCs, they first developed a fully-defined extracellular matrix for cardiomyocyte differentiation and maturation. A synthetic terpolymer scaffold was developed to aid the process. The team then identified specific hydrogel chemical and mechanical properties that accelerate cardiomyocyte maturation.
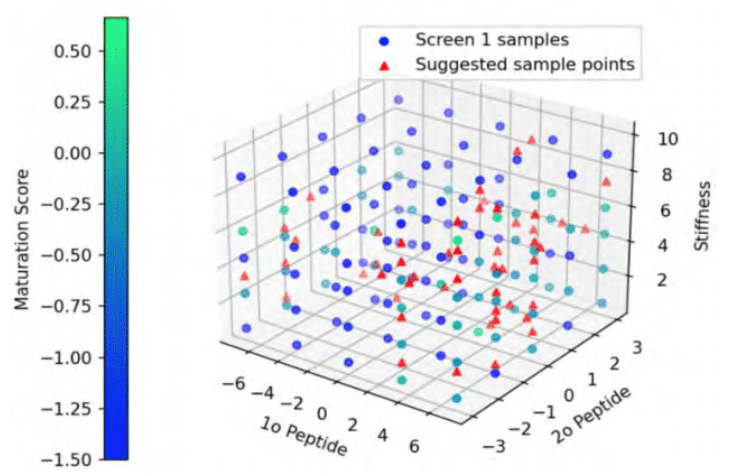
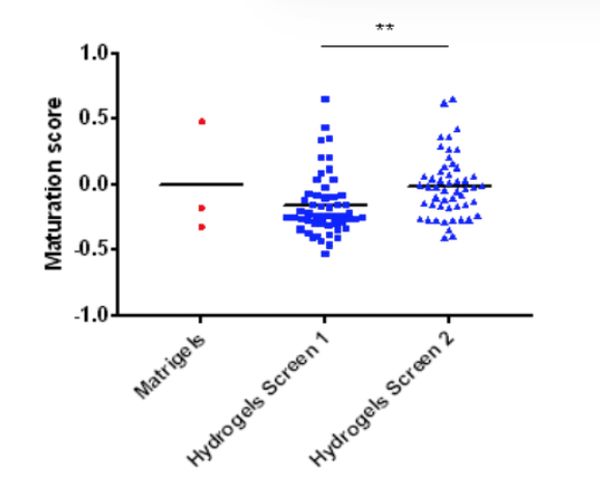
A multivariate analysis was completed to gauge the effect of the polyethylene glycol (PEG) hydrogel variables on CM survival. Cell-biomaterial interactions that enhance iPSC-CM maturation markers and phenotypes were identified. Through combinatorial screening of chemical and mechanical properties of the hydrogel library, the researchers identified peptides and stiffness conditions that provide robust CM differentiation with maturation on par with the gold standard, Matrigel. Native hyaluronic acid (HA) hydrogels were successfully used to support iPSC-CMs at ambient temperature and while maintaining functional metrics.
As a result of this work, prediction of the iPSC-derived cardiomyocyte potency has extended the multi-omics characterization to relate epigenetic and transcriptomic data, yielding insight in genomic loci that are implicated in differentiation potential from cardiac progenitor cells to cardiomyocytes. Six papers were published and three patents have been filed. The research team includes Maribella Domenech (University of Puerto Rico at Mayaguez); Timothy Kamp (University of Wisconsin-Madison); William Murphy (University of Wisconsin-Madison); and Madeline Torres-Lugo (University of Puerto Rico at Mayaguez).
Location
Atlanta, GeorgiaStart Year
Advanced Manufacturing
Advanced Manufacturing
Lead Institution
Core Partners
Fact Sheet
Outcome/Accomplishment
Researchers at the U.S. National Science Foundation (NSF)-funded Cell Manufacturing Technologies (NSF CMaT) Engineering Research Center (ERC), have developed a novel technique for generating cardiac cells derived from induced pluripotent stem cells (iPSCs). The team successfully identified genetic markers of atrial and ventricular cardiac fibroblast to enable discrimination of these populations. Right ventricular cardiomyocytes were then generated from iPSCs and validated for their molecular and functional properties. Finally, the team generated iPSC-derived pericytes from cardiac progenitor cells using constitutive activation of NOTCH1 signaling.
Location
Atlanta, GeorgiaStart Year
Advanced Manufacturing
Advanced Manufacturing
Lead Institution
Core Partners
Fact Sheet
Impact/benefits
: Cell therapies, especially stem cell and immune cell therapies, are revolutionizing the way we treat devastating, incurable, and chronic diseases, such as heart disease. Human-induced iPSCs hold great promise for reducing the mortality of cardiovascular disease by cellular replacement of infarcted cardiomyocytes (CMs). NSF CMaT’s work on iPSC-derived cardiac cells will impact how manufacturing develops in early-stage clinical implementation of these cells and other cell types that require differentiation. Specifically, NSF CMaT research enhances the scalability of manufacturing iPSC-derived cardiac cells and microtissues and improves the quality and maturity of the resulting cells for in vitro diagnostic and in vivo therapeutic applications.
Industry partners have already expressed interest in a variety of iPSC-derived cell types. Efforts to generate chamber-specific cell types are now underway, including atrial and ventricular cardiomyocytes and cardiac fibroblasts. While the focus of this work has been on cardiac cells primarily, the approaches and technologies developed will be relevant to other iPSC-derived cell types and iPSC-derived products, including iPSC-derived immune cells and neural cells.
Explanation/Background
The NSF CMaT research team, led by Sean Palecek at the University of Wisconsin-Madison, made significant progress in using materials-based approaches to improve the differentiation and maturation of iPSC-CMs. To generate cardiac pericyte-like cells from iPSCs, they first developed a fully-defined extracellular matrix for cardiomyocyte differentiation and maturation. A synthetic terpolymer scaffold was developed to aid the process. The team then identified specific hydrogel chemical and mechanical properties that accelerate cardiomyocyte maturation.
A multivariate analysis was completed to gauge the effect of the polyethylene glycol (PEG) hydrogel variables on CM survival. Cell-biomaterial interactions that enhance iPSC-CM maturation markers and phenotypes were identified. Through combinatorial screening of chemical and mechanical properties of the hydrogel library, the researchers identified peptides and stiffness conditions that provide robust CM differentiation with maturation on par with the gold standard, Matrigel. Native hyaluronic acid (HA) hydrogels were successfully used to support iPSC-CMs at ambient temperature and while maintaining functional metrics.
As a result of this work, prediction of the iPSC-derived cardiomyocyte potency has extended the multi-omics characterization to relate epigenetic and transcriptomic data, yielding insight in genomic loci that are implicated in differentiation potential from cardiac progenitor cells to cardiomyocytes. Six papers were published and three patents have been filed. The research team includes Maribella Domenech (University of Puerto Rico at Mayaguez); Timothy Kamp (University of Wisconsin-Madison); William Murphy (University of Wisconsin-Madison); and Madeline Torres-Lugo (University of Puerto Rico at Mayaguez).