CMaT ERC Achieves Integration of Imaging Modalities with Omics Characterization
Outcome/Accomplishment
Researchers with the National Science Foundation (NSF)-sponsored Cell Manufacturing Technologies (CMAT) Engineering Research Center (ERC), based at the Georgia Institute of Technology and the University of Georgia, successfully completed imaging of CAR-T cells in cell culture dishes using both differential phase contrast (DPC) and Fourier Ptychography Microscopy with 10x and 4x magnification.
Impact/Benefits
CAR-T cells afford great potential to manipulate the immune system and treat diseases such as large B-cell lymphoma. Yet challenges remain for the variability of sample preparation and establishing quality measures that are predictive of potency, safety, and consistency for manufacturing purposes.Deep cellular characterization provides an unprecedented level of information for cell manufacturing; however, the acquisition of multi-omics information is also challenging to reproduce. CMaT's cost-effective, high-throughput technologies are needed to serve as surrogate metrics to screen for Critical Quality Attributes (CQAs).
Explanation/Background
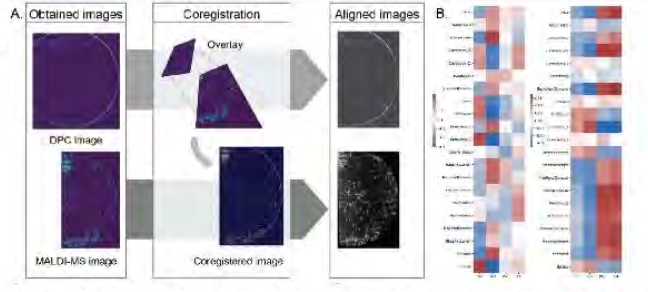
The CMaT team set out to engineer a label-free, non-destructive, low-cost imaging method to identify predictive measures of donor quality and cell potency that can be customized for non-adherent cells such as CAR-T cells. A secondary goal was to develop an analytical pipeline for evaluating spatial metabolic heterogeneity within multicellular contexts by integrating MALDI data with confocal imaging to provide single cell positional information.
The multi-modal co-registration of quantitative differential phase contrast (DPC) and MALDI mass spectrometry imaging was achieved to obtain a lipidomic profile associated with label-free morphology. A machine learning model was then built for cardiomyocyte differentiation that requires only an oxygen consumption rate measurement. Metrics derived from the time-series maximized accuracy to 93 percent after just 72 hours of monitoring. Phospholipid CQAs were identified as predictive of pluripotency loss in induced pluripotent stem cells (iPSCs) early in culture. The team also developed a potency assay based on the morphological response of microglia to interferon gamma (IFN-y) and tumor necrosis factor (TNF-a).
Location
Atlanta, GeorgiaStart Year
Advanced Manufacturing
Advanced Manufacturing
Lead Institution
Core Partners
Fact Sheet
Outcome/Accomplishment
Researchers with the National Science Foundation (NSF)-sponsored Cell Manufacturing Technologies (CMAT) Engineering Research Center (ERC), based at the Georgia Institute of Technology and the University of Georgia, successfully completed imaging of CAR-T cells in cell culture dishes using both differential phase contrast (DPC) and Fourier Ptychography Microscopy with 10x and 4x magnification.
Location
Atlanta, GeorgiaStart Year
Advanced Manufacturing
Advanced Manufacturing
Lead Institution
Core Partners
Fact Sheet
Impact/benefits
CAR-T cells afford great potential to manipulate the immune system and treat diseases such as large B-cell lymphoma. Yet challenges remain for the variability of sample preparation and establishing quality measures that are predictive of potency, safety, and consistency for manufacturing purposes.Deep cellular characterization provides an unprecedented level of information for cell manufacturing; however, the acquisition of multi-omics information is also challenging to reproduce. CMaT's cost-effective, high-throughput technologies are needed to serve as surrogate metrics to screen for Critical Quality Attributes (CQAs).
Explanation/Background
The CMaT team set out to engineer a label-free, non-destructive, low-cost imaging method to identify predictive measures of donor quality and cell potency that can be customized for non-adherent cells such as CAR-T cells. A secondary goal was to develop an analytical pipeline for evaluating spatial metabolic heterogeneity within multicellular contexts by integrating MALDI data with confocal imaging to provide single cell positional information.
The multi-modal co-registration of quantitative differential phase contrast (DPC) and MALDI mass spectrometry imaging was achieved to obtain a lipidomic profile associated with label-free morphology. A machine learning model was then built for cardiomyocyte differentiation that requires only an oxygen consumption rate measurement. Metrics derived from the time-series maximized accuracy to 93 percent after just 72 hours of monitoring. Phospholipid CQAs were identified as predictive of pluripotency loss in induced pluripotent stem cells (iPSCs) early in culture. The team also developed a potency assay based on the morphological response of microglia to interferon gamma (IFN-y) and tumor necrosis factor (TNF-a).