Selective Electrosorption for Silica Removal and Scaling Prevention
Outcome/Accomplishment
A batch flow-through electrosorption system that successfully removes dissolved silica has been developed by researchers at the National Science Foundation (NSF)-funded Nanotechnology Enabled Water Treatment (NEWT) Engineering Research Center (ERC) headquartered at Rice University. The team functionalized an activated carbon cloth electrode and enhanced its silica removal capacity and selectivity during electrosorption. Several silica nanoadsorbents were assessed and calcined LDH (aluminum magnesium layered double hydroxide (Mg/Al-LDH)) was identified as offering the highest silica adsorption capacity and selectivity for anode functionalization.
Impact/Benefits
Dissolved silica is a major concern in a broad range of industrial processes due to scale formation, which significantly compromises process performance. Silica scaling is especially prevalent in reverse osmosis (RO) desalination, resulting in both performance loss and limited water recovery. Current technologies demonstrate shortcomings in their removal efficacy, selectivity, scalability, environmental sustainability, and economic competitiveness. Unlike traditional adsorption processes for silica removal, electrosorption offers easy and chemical free material regeneration. NEWT's work on silica removal via electrosorption enables a more economic, selective, and effective technology for scaling mitigation.
Explanation/Background
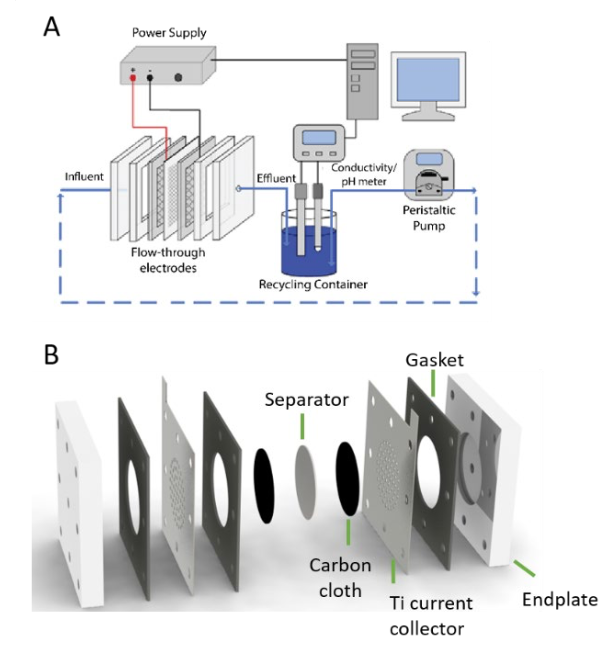
The design of an electrode capable of recognizing and selectively interacting with silica was essential to the development of the proposed electrosorption technology. To test selective nanomaterials for silica adsorption, a thin-film coating of nanomaterials was applied over the anode pores to enhance silica-selective partitioning and transport into the pores for electrosorption. NEWT suggested aluminum-based nanoparticles as primary candidates for the anode surface coating. Silica-selective ion exchange resins, which have been commercially proven to be effective for silica removal, were also tested as an alternative surface coating material. Tetrahedral-specific receptors, such as sulfate and phosphate binding receptors, were investigated for their ability to exploit the molecular geometry of the silicate ion. These tests helped to optimize the mass loading and coating thickness of the material on the anode. The resulting system enabled a silica selective electrode by functionalizing a commercial activated-carbon cloth with aluminum-based nanoparticles.
To assess system performance, the team successfully constructed a flow-through electrosorption setup and evaluated the pH increase of the cathode under different external voltages. Optimal operating voltage minimizes anodic oxidation reactions, while maintaining elevated pH at the cathode and large amounts of electrosorption. The team found that at 1 volt (V), there is sufficient electrosorption while minimizing the occurrence of anodic reactions. A significant pH increase at the cathode in flow-through electrosorption conditions was observed, converting monosilicic acid to the silicate ion in a chemical-free manner. The silicate ion is selectively electrosorbed at the anode, which is strategically designed to maximize silica removal efficacy and selectivity.
Four types of synthesized, second-generation silica nanoadsorbents based on layered double hydroxide (LDH) were also tested and compared: aluminum hydroxide (Al(OH)3), aluminum magnesium layered double hydroxide (Mg/Al-LDH), calcined Al(OH)3, and calcined Mg/Al-LDH. Calcined LDH exhibits the highest silica adsorption capacity; and, therefore, it will be selected as a potential material for anode modification. Future work will compare the effectiveness of different anode functionalization methods: in situ development of nanoadsorbents and coating nanoadsorbents through electrodeposition.
Location
Houston, Texaswebsite
Start Year
Energy and Sustainability
Energy, Sustainability, and Infrastructure
Lead Institution
Core Partners
Fact Sheet
Outcome/Accomplishment
A batch flow-through electrosorption system that successfully removes dissolved silica has been developed by researchers at the National Science Foundation (NSF)-funded Nanotechnology Enabled Water Treatment (NEWT) Engineering Research Center (ERC) headquartered at Rice University. The team functionalized an activated carbon cloth electrode and enhanced its silica removal capacity and selectivity during electrosorption. Several silica nanoadsorbents were assessed and calcined LDH (aluminum magnesium layered double hydroxide (Mg/Al-LDH)) was identified as offering the highest silica adsorption capacity and selectivity for anode functionalization.
Location
Houston, Texaswebsite
Start Year
Energy and Sustainability
Energy, Sustainability, and Infrastructure
Lead Institution
Core Partners
Fact Sheet
Impact/benefits
Dissolved silica is a major concern in a broad range of industrial processes due to scale formation, which significantly compromises process performance. Silica scaling is especially prevalent in reverse osmosis (RO) desalination, resulting in both performance loss and limited water recovery. Current technologies demonstrate shortcomings in their removal efficacy, selectivity, scalability, environmental sustainability, and economic competitiveness. Unlike traditional adsorption processes for silica removal, electrosorption offers easy and chemical free material regeneration. NEWT's work on silica removal via electrosorption enables a more economic, selective, and effective technology for scaling mitigation.
Explanation/Background
The design of an electrode capable of recognizing and selectively interacting with silica was essential to the development of the proposed electrosorption technology. To test selective nanomaterials for silica adsorption, a thin-film coating of nanomaterials was applied over the anode pores to enhance silica-selective partitioning and transport into the pores for electrosorption. NEWT suggested aluminum-based nanoparticles as primary candidates for the anode surface coating. Silica-selective ion exchange resins, which have been commercially proven to be effective for silica removal, were also tested as an alternative surface coating material. Tetrahedral-specific receptors, such as sulfate and phosphate binding receptors, were investigated for their ability to exploit the molecular geometry of the silicate ion. These tests helped to optimize the mass loading and coating thickness of the material on the anode. The resulting system enabled a silica selective electrode by functionalizing a commercial activated-carbon cloth with aluminum-based nanoparticles.
To assess system performance, the team successfully constructed a flow-through electrosorption setup and evaluated the pH increase of the cathode under different external voltages. Optimal operating voltage minimizes anodic oxidation reactions, while maintaining elevated pH at the cathode and large amounts of electrosorption. The team found that at 1 volt (V), there is sufficient electrosorption while minimizing the occurrence of anodic reactions. A significant pH increase at the cathode in flow-through electrosorption conditions was observed, converting monosilicic acid to the silicate ion in a chemical-free manner. The silicate ion is selectively electrosorbed at the anode, which is strategically designed to maximize silica removal efficacy and selectivity.
Four types of synthesized, second-generation silica nanoadsorbents based on layered double hydroxide (LDH) were also tested and compared: aluminum hydroxide (Al(OH)3), aluminum magnesium layered double hydroxide (Mg/Al-LDH), calcined Al(OH)3, and calcined Mg/Al-LDH. Calcined LDH exhibits the highest silica adsorption capacity; and, therefore, it will be selected as a potential material for anode modification. Future work will compare the effectiveness of different anode functionalization methods: in situ development of nanoadsorbents and coating nanoadsorbents through electrodeposition.