Biofilm Eradication and Selective Microbial Control Using Phages Conjugated with Superparamagnetic Nanoparticles (SNPs)
Outcome/Accomplishment
Researchers with the National Science Foundation's (NSF) Nanotechnology Enabled Water Treatment (NEWT) Engineering Research Center (ERC) headquartered at Rice University have used combined experimental and computational approaches to demonstrate the importance of nano-magnetite particle size control in enhancing the bottom-up biofilm eradication capability of phage-nanocomposite conjugates, or PNCs. The experiments helped to discern the dose-response relationship of bacterial biofilm to polyvalent phage treatment while establishing a high-throughput method for enriching and screening phages with high biofilm removal potential using superparamagnetic nanoparticle (SNP)-conjugated bacterial hosts.
Impact/Benefits
Biofilms are pervasive and problematic agglomerations of microorganisms that hinder water treatment and reuse systems. Biofilms may shelter pathogenic or other problematic bacteria, enable multidrug resistant "superbugs," or enhance microbial-induced corrosion. They are especially difficult to eradicate due to hindered penetration of disinfectants. And their associated biofouling can increase the costs and complexity of membrane-based water treatment processes. NEWT's studies are aimed at optimizing magnetic nano-phage conjugates (MNPCs) for bottom-up biofilm eradication and selective control of target bacteria in water treatment, distribution, and/or storage systems.
Explanation/Background
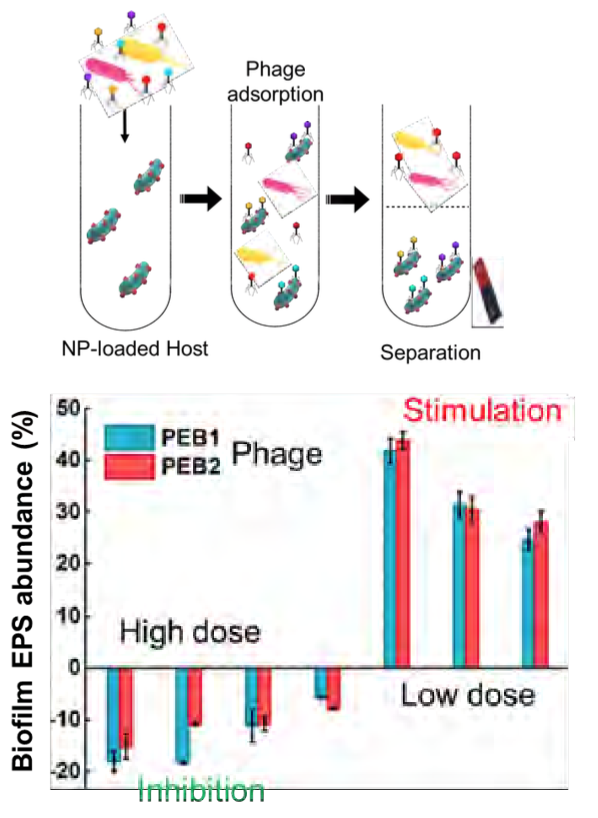
NEWT post-doctoral researcher, Pingfeng Yu, spearheaded the biofilm eradication project. A multiple-host isolation method was applied to screen and enrich the polyvalent phages without prior identification of all target bacteria. To enhance biofilm disruption, Yu's team planned to test depolymerase activity based on enzymatic activity. Synthetic ferrimagnetic magnetite magnetic nanoparticles (Fe3O4-MNP) of different sizes were applied followed by surface coating with APTES or Chitosan. By covalently conjugating phages onto the nanoparticles (NPs) with crosslinkers, the team evolved the proper orientation to enhance infectivity. Synthetic NPs (SNPs) were conjugated onto a bacterial surface to ensure phage penetration into the biofilm and enable magnetic phage extraction.
The team investigated how NP size influences the phage propagation and biofilm removal and found that NP size does affect phage propagation patterns. The bottom-up biofilm-eradication capability of different magnetic nanoparticle conjugates (MNPCs) on lab-cultured biofilms was also tested. Finally, a semi-empirical biofilm-phage model was calibrated using the experimental results to discern horizontal and vertical penetration of differently-sized nano-conjugates.
The research revealed that smaller NPCs enhanced biofilm bottom clearance with significant horizontal dispersion, while larger NPCs exerted more significant vertical biofilm disruption. The stochastic spatial model infers that biofilm response to phage infection exhibits a hormesis phenomenon with a high dose of phages removing biofilm, while a low dose of phages simulates biofilm formation. This discovery highlights the importance of efficient phage delivery to eradicate biofilms, which might be achieved by loading phages onto SNPs and enhancing their penetration all the way to the base of the biofilm using a weak magnetic field. NEWT's results were published in a 2019 Environmental Science: Nano cover article entitled "Bottom-Up Biofilm Eradication Using Bacteriophage-Loaded Magnetic Nanocomposites: A Computational and Experimental Study."
Location
Houston, Texaswebsite
Start Year
Energy and Sustainability
Energy, Sustainability, and Infrastructure
Lead Institution
Core Partners
Fact Sheet
Outcome/Accomplishment
Researchers with the National Science Foundation's (NSF) Nanotechnology Enabled Water Treatment (NEWT) Engineering Research Center (ERC) headquartered at Rice University have used combined experimental and computational approaches to demonstrate the importance of nano-magnetite particle size control in enhancing the bottom-up biofilm eradication capability of phage-nanocomposite conjugates, or PNCs. The experiments helped to discern the dose-response relationship of bacterial biofilm to polyvalent phage treatment while establishing a high-throughput method for enriching and screening phages with high biofilm removal potential using superparamagnetic nanoparticle (SNP)-conjugated bacterial hosts.
Location
Houston, Texaswebsite
Start Year
Energy and Sustainability
Energy, Sustainability, and Infrastructure
Lead Institution
Core Partners
Fact Sheet
Impact/benefits
Biofilms are pervasive and problematic agglomerations of microorganisms that hinder water treatment and reuse systems. Biofilms may shelter pathogenic or other problematic bacteria, enable multidrug resistant "superbugs," or enhance microbial-induced corrosion. They are especially difficult to eradicate due to hindered penetration of disinfectants. And their associated biofouling can increase the costs and complexity of membrane-based water treatment processes. NEWT's studies are aimed at optimizing magnetic nano-phage conjugates (MNPCs) for bottom-up biofilm eradication and selective control of target bacteria in water treatment, distribution, and/or storage systems.
Explanation/Background
NEWT post-doctoral researcher, Pingfeng Yu, spearheaded the biofilm eradication project. A multiple-host isolation method was applied to screen and enrich the polyvalent phages without prior identification of all target bacteria. To enhance biofilm disruption, Yu's team planned to test depolymerase activity based on enzymatic activity. Synthetic ferrimagnetic magnetite magnetic nanoparticles (Fe3O4-MNP) of different sizes were applied followed by surface coating with APTES or Chitosan. By covalently conjugating phages onto the nanoparticles (NPs) with crosslinkers, the team evolved the proper orientation to enhance infectivity. Synthetic NPs (SNPs) were conjugated onto a bacterial surface to ensure phage penetration into the biofilm and enable magnetic phage extraction.
The team investigated how NP size influences the phage propagation and biofilm removal and found that NP size does affect phage propagation patterns. The bottom-up biofilm-eradication capability of different magnetic nanoparticle conjugates (MNPCs) on lab-cultured biofilms was also tested. Finally, a semi-empirical biofilm-phage model was calibrated using the experimental results to discern horizontal and vertical penetration of differently-sized nano-conjugates.
The research revealed that smaller NPCs enhanced biofilm bottom clearance with significant horizontal dispersion, while larger NPCs exerted more significant vertical biofilm disruption. The stochastic spatial model infers that biofilm response to phage infection exhibits a hormesis phenomenon with a high dose of phages removing biofilm, while a low dose of phages simulates biofilm formation. This discovery highlights the importance of efficient phage delivery to eradicate biofilms, which might be achieved by loading phages onto SNPs and enhancing their penetration all the way to the base of the biofilm using a weak magnetic field. NEWT's results were published in a 2019 Environmental Science: Nano cover article entitled "Bottom-Up Biofilm Eradication Using Bacteriophage-Loaded Magnetic Nanocomposites: A Computational and Experimental Study."