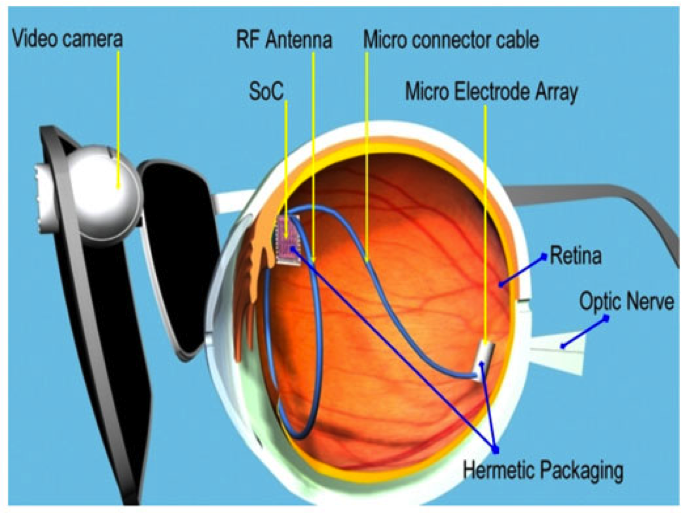
USC’s Biomimetic MicroElectronic Systems ERC – BMES has successfully conducted clinical testing of a second-generation implantable prosthetic retina, the Argus II Retinal Prosthesis System, developed in conjunction with a device manufacturer, Second Sight Medical Products. The device transmits images from a small, glasses-mounted camera to a 60-pixel microelectrode array implanted on a patient’s damaged retina. The array sends signals, via the optic nerve, that the brain interprets visually. The implant allows people with retinitis pigmentosa who are completely blind to locate objects, detect movement, improve orientation and mobility skills, and discern shapes such as large letters
Potential market value of this technology worldwide depends on improvements in the power, reliability, and biocompatibility of the device and its eventual pricing, but the market is clearly in the billions of dollars. NSF’s continued funding of this ERC is expected to enable near-normal sight through a fully implantable prosthesis with high reliability.
In January 2013, the U. S. Food and Drug Administration (FDA) granted market approval for clinical use to the Argus II, the first bionic eye to be approved for patients in the United States.
USC’s Biomimetic MicroElectronic Systems ERC – BMES has successfully conducted clinical testing of a second-generation implantable prosthetic retina, the Argus II Retinal Prosthesis System, developed in conjunction with a device manufacturer, Second Sight Medical Products. The device transmits images from a small, glasses-mounted camera to a 60-pixel microelectrode array implanted on a patient’s damaged retina. The array sends signals, via the optic nerve, that the brain interprets visually. The implant allows people with retinitis pigmentosa who are completely blind to locate objects, detect movement, improve orientation and mobility skills, and discern shapes such as large letters
Potential market value of this technology worldwide depends on improvements in the power, reliability, and biocompatibility of the device and its eventual pricing, but the market is clearly in the billions of dollars. NSF’s continued funding of this ERC is expected to enable near-normal sight through a fully implantable prosthesis with high reliability.
In January 2013, the U. S. Food and Drug Administration (FDA) granted market approval for clinical use to the Argus II, the first bionic eye to be approved for patients in the United States.